FDA Accelerates Full Approval of Pfizer-BioNTech Vaccine as Delta Variant Surges. By Abigail Abrams and Brian Bennett August 4 2021 638 PM EDT T he Food and Drug Administration FDA has begun.
Vaccinations For Adolescents Flyer Health Mil
Article Pfizer and BioNTech vaccine 86 effective among older people after third dose.
Pfizer approval letter. PFE today announced that the European Commission EC has approved RUXIENCE rituximab a monoclonal antibody mAb and biosimilar to MabThera rituximab for the treatment of non-Hodgkins lymphoma NHL chronic lymphocytic leukemia CLL rheumatoid arthritis RA granulomatosis with polyangiitis GPA and. Drug Approval Package. NYSEPFE today announced that the US.
Article Valneva begins UK rolling submission for COVID-19 vaccine. Accordingly the ANDA is approved effective on the date of this letter. The Office of Bioequivalence has determined your Sildenafil Citrate Tablets 25 mg 50 mg and 100 mg to be bioequivalent and therefore therapeutically equivalent to the reference listed drug RLD Viagra Tablets of Pfizer Ireland Pfizer.
Permanent US approval for Pfizer and BioNTech vaccine. Amit Patel Pfizer Inc. New Drug Application and for Discontinuation of Commercial Availability of Mylotarg for Relapsed Acute Myeloid Leukemia IMPORTANT PRESCRIBING INFORMATION June 21 2010 Dear Healthcare Professional Re.
Elisa Harkins 500 Arcola Road Collegeville PA 19426 Dear Ms. Please refer to your supplemental new drug application dated and received August 14 2019 submitted under section 505b of the Federal Food Drug and Cosmetic Act FDCA for Ibrance palbociclib capsules. PFE today announced the European Commission EC has approved ZIRABEV for the treatment of metastatic carcinoma of the colon or rectum metastatic breast cancer unresectable advanced metastatic or recurrent non-small cell lung cancer NSCLC advanced andor metastatic renal cell cancer and persistent recurrent or metastatic carcinoma of the cervix12.
Pfizer Global Regulatory Affairs 445 Eastern Point Road Groton CT 06340. On December 11 2020 the US. Persons with disabilities having problems accessing the PDF files below may call 301 796-3634 for assistance.
NEW YORK-- BUSINESS WIRE-- Pfizer Inc. Mylotarg gemtuzumab ozogamicin for Injection for patients with CD33 acute myeloid. Federal regulators are winding down the process of licensing Pfizers two-dose coronavirus vaccine setting up an approval possibly by Monday and possibly kicking off a wave of new mandates.
The new target is. Food and Drug Administration issued the first emergency use authorization EUA for a vaccine for the. Dear Healthcare Professional Letter Pfizer Prepares for Voluntary Withdrawal of US.
June 10 2021 Approval Letter - PREVNAR 20 June 8 2021 Summary Basis for Regulatory Action - PREVNAR 20 Approval History Letters Reviews and Related Documents - PREVNAR 20. On February 4 2020 pursuant to Section 564b1C of the Act the. OUR SCIENCE An Open Letter from Pfizer Chairman and CEO Albert Bourla I know there is a great deal of confusion regarding exactly what it will take to ensure its development and approval and given the critical public health considerations and the importance of transparency I would like to provide greater clarity around the development timelines for Pfizers and our partner BioNTechs.
Information about the Pfizer-BioNTech COVID-19 vaccine. The Food and Drug Administration is planning to authorize full approval of the Pfizer-BioNTech two-dose COVID-19 vaccine on Monday according to The New York Times. Pfizer and its German manufacturing partner BioNTech have begun their application to gain full approval of its Covid-19 vaccine from the.
Additional reference is made to our Safety Labeling Change Notification letter dated July 15. 235 East 42nd Street New York NY 10017. BL 1257420 BLA APPROVAL BioNTech Manufacturing GmbH August 23 2021 Attention.
TGA provisionally approves Pfizer COVID-19 vaccine 25 January 2021 The Therapeutic Goods Administration TGA has granted provisional approval to Pfizer Australia Pty Ltd for its COVID-19 vaccine COMIRNATY making it the first COVID-19 vaccine to receive regulatory approval in Australia. APPROVAL LABELING We have completed our review of this application as amended. 2019 2 3 May 10 2021 Pfizer Inc.
FDA Approval Letter and Labeling. More on this story. More than 200 million doses of the Pfizer vaccine have been administered in the US since December.
DrugsFDA information available about RUXIENCE. Food and Drug Administration approved MYLOTARG gemtuzumab ozogamicin for adults with newly diagnosed CD33-positive acute myeloid leukemia AML and adults and children 2 years and older with relapsed or refractory CD33-positive AML1 MYLOTARG is the first therapy with an indication that includes pediatric AML. Brian Kemp said in a tweet that he sent a letter.
It is approved effective on the date of this letter for use as recommended in the. Your dissolution testing.
U K Becomes First Country To Approve Pfizer Biontech Covid 19 Vaccine

Pentagon To Mandate Covid 19 Vaccine For Military After Full Pfizer Approval The Boston Globe

Pfizer Biontech Covid Vaccine Approved By Panel Clearing Way For Fda Authorization For Emergency Use As It Happened Us News The Guardian
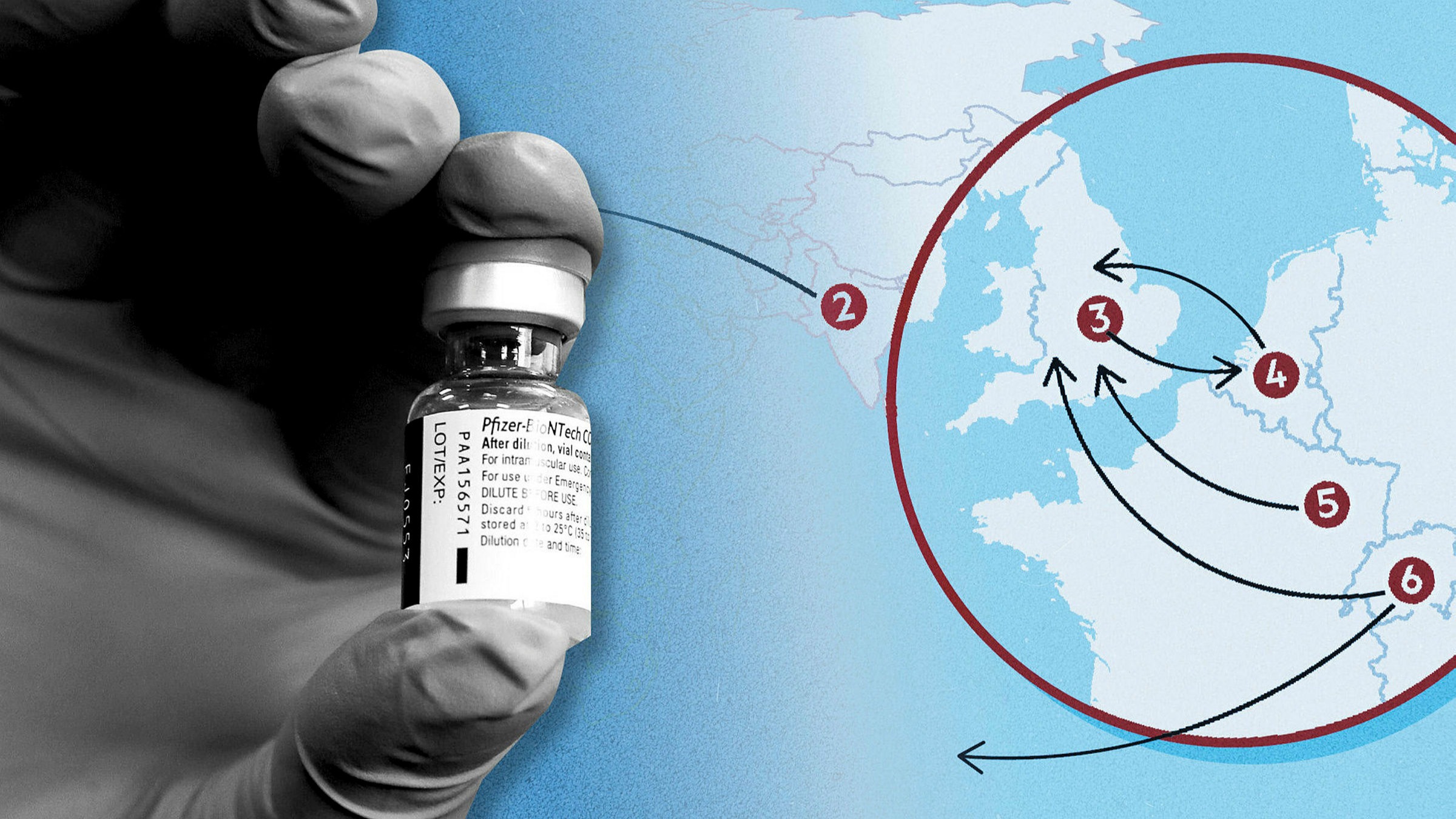
Eu Threat To Vaccine Exports Exposes Mutual Risks To Global Supply Chain Financial Times
Coronavirus Live Germans Told To Take Pfizer Or Moderna As Second Dose After Astrazeneca For Better Protection World News The Guardian
Pfizer Biontech Cut Back Vaccine Deliveries To Eu At Short Notice Europe News And Current Affairs From Around The Continent Dw 15 01 2021
Covid 19 Vaccines As Early As Friday In Us Fda Advisor Says
Eu Countries Decry Very Short Notice Of Delay In Delivery Of Pfizer Vaccine Financial Times

Pfizer Inc Pfizer And Biontech To Co Develop Potential Covid 19 Vaccine

Health Experts Welcome Full Approval Of Pfizer Covid 19 Vaccine In Coming Weeks The Boston Globe
Uk Regulator Issues Allergy Warning On Pfizer Biontech Vaccine Financial Times

Pfizer Biontech Team Up To Advance Covid 19 Vaccine Development

Pune Man Writes To Pfizer Ceo Asking For Covid Vaccine In India Gets Immediate Response Cities News
German Coronavirus Vaccine Inventor Being Investigated Science In Depth Reporting On Science And Technology Dw 10 03 2021

Fda Expected To Convert Pfizer And Biontech Covid 19 Vaccine Eua To Full Approval Within Months
National Healthcare Associations Praise Fda Emergency Use Approval Of Pfizer Vaccine Healthcare Innovation
Hopes Rise For End Of Pandemic As Pfizer Says Vaccine Has 90 Efficacy Coronavirus The Guardian

Komentar