The FDA approved a drug for the treatment of Alzheimers under the accelerated approval pathway which can be used for a drug for a serious or life-threatening illness that provides meaningful. After approval Biogen set an average price of 56000 a year far higher than what an influential nonprofit that reviews drug pricing said would be cost effective.
Fda Approves Alzheimer S Drug Aduhelm But Concerns Raised About Cost Effectiveness
To get it in your inbox sign up for free.

Alzheimer's drug fda cost. Een luisterend oor of persoonlijk advies. The medication developed by Biogen will be marketed with the name Aduhelm for a. FDAs Approval of Biogens New Alzheimers Drug Has Huge Cost Implications for Medicare and Beneficiaries.
Daar zorgen wij voor. First this is a biologic drug and Biogen views it as a specialty drug so given the prices of such specialty drugs in recent years this high price was not atypical. New Alzheimers drug aducanumab.
Advertentie Financiële ondersteuning voor onderzoek of het luiden van de noodklok bij dementiezorg. And given those limited options it will certainly be a massive money-maker for its developer Biogen which has already set the list price for a years worth of Aduhelm at 56000. The Food and Drug Administration approved the first new drug for Alzheimers disease in nearly 20 years on Monday a drug which analysts have estimated could cost between 30000 to 50000 a year.
Safe and Effective with 5 Natural Ingredients. Biogen on Tuesday faced tough questions from Wall Street analysts over the 56000 annual cost of its newly approved Alzheimers drug Aduhelm. Advertentie Financiële ondersteuning voor onderzoek of het luiden van de noodklok bij dementiezorg.
Made in the USA. Clinically Proven Natural Medication Helps Protect Against Alzheimers. At a wholesale price of 56000 per year the company exceeded some Wall Street expectations and greatly surpassed the 8300 threshold that.
Today FDA approved Aduhelm aducanumab to treat patients with Alzheimers disease using the Accelerated Approval pathway under which the FDA approves a drug. FDA approves an Alzheimers drug 0145 A version of this story appeared in CNNs What Matters newsletter. A debate between FDA advisory committee members and FDA officials in top-tier journal New England Journal of Medicine NEJM reveals the controversy at the core of the FDAs approval of Biogens Alzheimers drug aducanumab Aduhelm.
Een luisterend oor of persoonlijk advies. According to the FDA advisory committee members The FDAs decision is at odds with the evidence and with the agencys biostatistical review. Juliette Cubanski Follow jcubanski on.
Safe and Effective with 5 Natural Ingredients. Cost side effects timeline and other questions answered Biogen announced in early June that the wholesale cost of treatment --. The Food and Drug Administration gave the green light to a controversial new Alzheimers drug -- despite objections from some of its own experts according to.
Many experts were expecting a price around 10000 based on current Alzheimers treatments but honestly the high price was not a shock to me. Made in the USA. Clinically Proven Natural Medication Helps Protect Against Alzheimers.
The US Food and Drug Administration FDA approved a treatment to slow down the progression of Alzheimers disease. Daar zorgen wij voor.
Fda Approves Controversial Alzheimer S Drug

Fda Approves Controversial Alzheimer S Drug From Biogen Epm Magazine

Alzheimers Q A Will The New Fda Approved Alzheimer S Drug Be Available Soon Health Fitness Theadvocate Com

Biogen S Alzheimer S Drug How Realistic Is A Rollout In The Uk Epm Magazine

5 Types Of Medication To Treat Difficult Alzheimer S Behaviors

Biogen Will Charge 56 000 A Year For Alzheimer S Drug
Biogen S 56 000 A Year Alzheimer S Drug Reignites Us Debate Over Costs Financial Times

Aduhelm Becomes First Alzheimer S Drug In 20 Years To Get Us Approval Here S What You Need To Kno The New Indian Express
First Drug To Slow Alzheimer S Disease Poised For Approval The Independent
/cdn.vox-cdn.com/uploads/chorus_image/image/69417246/merlin_98124000.7.jpg)
Fda Approves Much Debated Alzheimer S Drug Panned By Experts Chicago Sun Times

Clinicians View New Alzheimer S Drug With Guarded Optimism
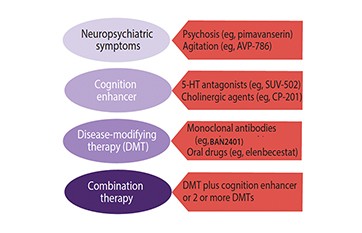
Alzheimer S Disease Drug Development Emerging Therapies Practical Neurology
Biogen Alzheimer S Drug Will Exceed The 56 000 List Price For Many Analysis Says Wsj

Roche Is Discussing Alzheimer S Drug With Fda Following Regulator S Controversial Approval Of Biogen S Aduhelm Ceo Says
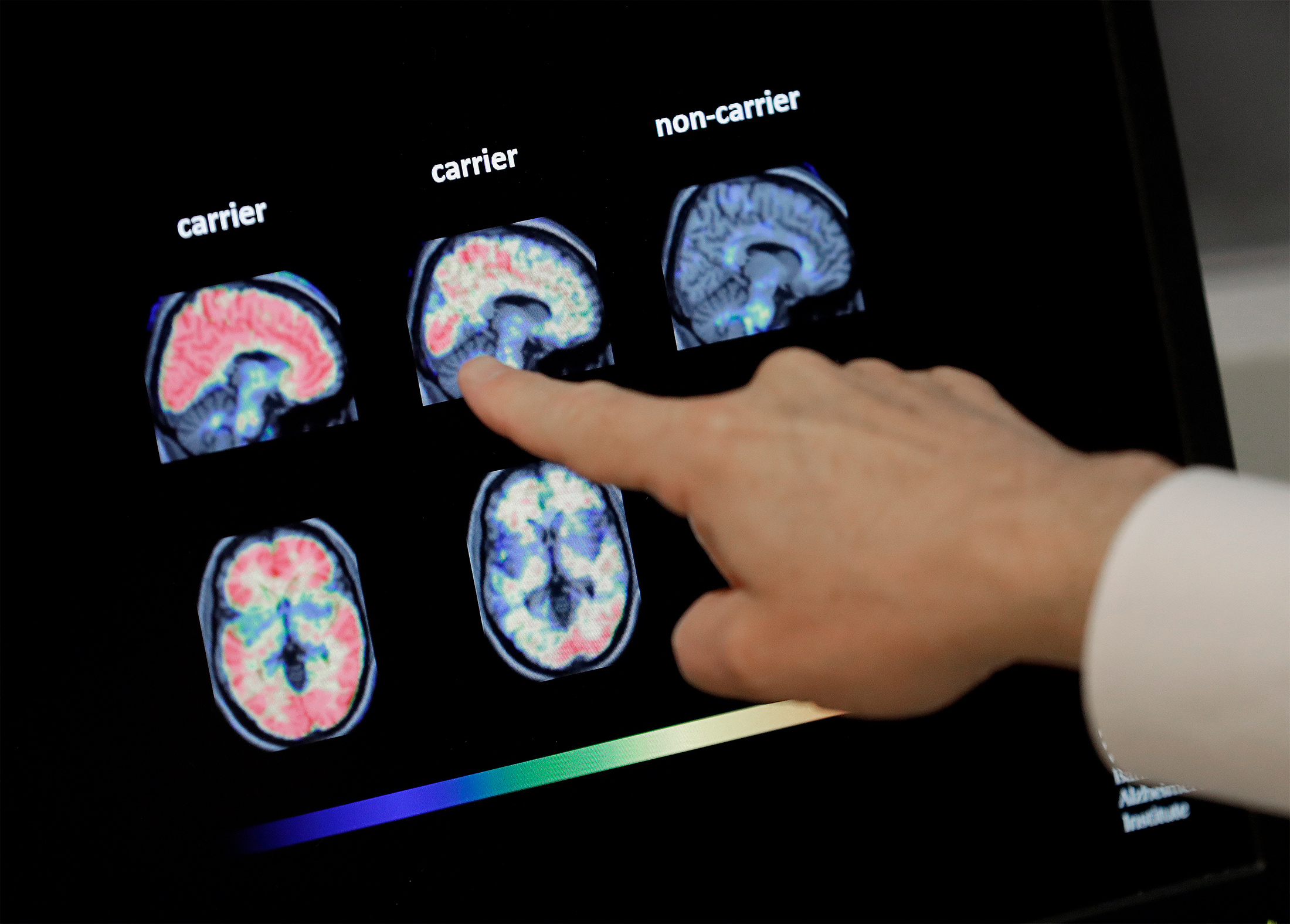
Biogen S New Alzheimer S Drug Faces Hurdles Reaching Patients Even If It S Approved Bloomberg

Fda Approves New Alzheimer S Disease Drug Bucks County Man Phil Gutis Discusses Experience In Clinical Trial 6abc Philadelphia

Fda Approves First New Alzheimer S Drug In Nearly 20 Years Health News Us News

Fda Approves First New Alzheimer S Drug In Almost 20 Years Alzheimer S The Guardian

Alzheimer S Drug Biogen Hopes Controversial Aducanumab Gets Fda Approval Bloomberg
/cloudfront-us-east-1.images.arcpublishing.com/gray/37QDV5VQVFDL7LTMHCGBWGWNRQ.PNG)

Komentar